Dr. Sabzali Javadov
Contact Info
Education
- MD 1983, Medicine, Russian Medical Univ., Moscow, Russia
- Ph.D. 1986, Cardiology, Cardiology Research Center, Moscow, Russia
- Training 1987, Medicine, Magdeburg Medical Academy, Germany
- Training 1991-92, Cardiology, National Institute of Cardiology, Hungary
- Training 1997-2001, Cardiology, University of Bristol, England
RESEARCH INTEREST
My laboratory elucidates mitochondria-mediated mechanisms of cardiac dysfunction during coronary heart diseases such as myocardial infarction (MI) and heart failure. MI /ischemia-reperfusion (IR) injury is a complex process that includes several cell death mechanisms such as apoptosis, necrosis, pyroptosis, necroptosis, autophagy, ferroptosis, among others. The contributions of these mecha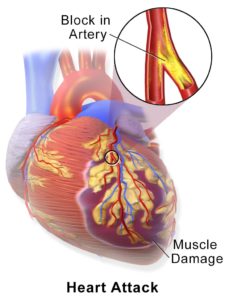 nisms to cell death/cardiac dysfunction depend on the severity and duration of the oxidative and energy stresses induced by MI/IR. Understanding the contributions of cell death types at different stages of post-MI/IR injury is important for precisely targeting a specific death mechanism. Mitochondria are involved in the pathogenesis of MI/IR injury and actively participate in all types of cell death mechanisms. However,
nisms to cell death/cardiac dysfunction depend on the severity and duration of the oxidative and energy stresses induced by MI/IR. Understanding the contributions of cell death types at different stages of post-MI/IR injury is important for precisely targeting a specific death mechanism. Mitochondria are involved in the pathogenesis of MI/IR injury and actively participate in all types of cell death mechanisms. However,
 limited knowledge of the mechanisms underlying mitochondria-mediated cell death obscures the development of new mitochondria-targeted cardioprotectors. One of our research interests is to clarify the role of mitochondria in ferroptosis, a recently discovered iron-dependent programmed cell death mechanism, during cardiac IR. Elucidation of the mitochondria-mediated mechanisms is important for timely restoration of mitochondrial function and cell survival during MI/IR injury. Thus, our main goal is to investigate molecular mechanisms underlying mitochondria-mediated cell death during MI/IR injury and develop new therapeutic strategies that could prevent myocardial injury and improve clinical outcomes in patients with post-MI/IR injury and heart failure through targeting mitochondria.
limited knowledge of the mechanisms underlying mitochondria-mediated cell death obscures the development of new mitochondria-targeted cardioprotectors. One of our research interests is to clarify the role of mitochondria in ferroptosis, a recently discovered iron-dependent programmed cell death mechanism, during cardiac IR. Elucidation of the mitochondria-mediated mechanisms is important for timely restoration of mitochondrial function and cell survival during MI/IR injury. Thus, our main goal is to investigate molecular mechanisms underlying mitochondria-mediated cell death during MI/IR injury and develop new therapeutic strategies that could prevent myocardial injury and improve clinical outcomes in patients with post-MI/IR injury and heart failure through targeting mitochondria.
Our research aims to:
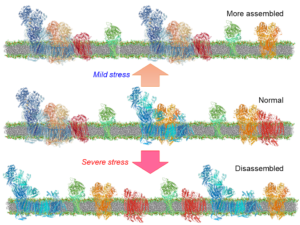
1. Investigate the role of respiratory supercomplexes (SCs), large multiprotein complexes containing individual electron transport chain (ETC) complexes, in the pathogenesis of cardiac IR injury. Also, we assess the mechanisms of SC formation, particularly the role of ETC complexes and ANT in SC assembly in cardiac cells.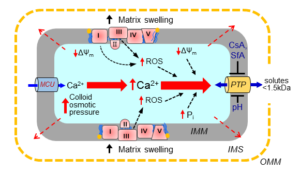
2. Determine the role of the inner mitochondrial membrane in mitochondria-targeted cardioprotection against cardiac IR injury. These studies are aimed to investigate the crosstalk between permeability transition pore (PTP)-induced swelling, OPA1 proteolytic cleavage, and MICOS proteins in mediating cell death signaling during cardiac IR injury. Also, we elucidate the mechanisms of ferroptosis, a recently discovered programmed cell death, in the heart/cultured cardiomyocytes in response to external stimuli.
3. Develop a biophysical model of mitochondrial swelling to describe the transition from reversible to irreversible swelling of cardiac mitochondria in response to external stimuli. We develop the in silico modeling from a simple kinetic model to a complex model that takes into consideration the dynamics of mitochondrial ion diffusion, kinetic factors, membrane potential, and membrane mechanical properties and thus, provides a solid foundation for application of new developed mathematical tools.
Funding: National Institute of General Medical Sciences, National Institutes of Health, USA
Lab Personnel: Sehwan Jang (Postdoctoral Researcher), Esteban Ayala (Research Assistant),
Xavier Chapa (PhD student), Keishla Rodriguez (PhD student); Jorge Garcia (Undergraduate student)
PAST STUDENTS
Rebecca Parodi, PhD-2018
Griselle Barreto, PhD-2015
CURRENT STUDENTS
Keishla Rodríguez Graciani
Undergraduate
Jorge García Báez, UPR, Río Piedras
Ivana Chávez, UPR, Río Piedras
GRANTS
09/01/22-08/31/26
Mitochondria-mediated mechanisms of ferroptosis in response to cardiac ischemia-reperfusion injury
2006477 NSF
06/01/20-06-30-24
Biophysical musltiscale modeling of mitochondrial swelling
PR Science, Technology and Research Trust
08/21/22-07/31/23
New mitochondria-targeted therapy for myocardial infaction
SCIENTIFIC COLLABORATORS
Dr. Jason, University of Michigan, MI
PUBLICATIONS
- Sparvero L, Tian H, Amoscato AA, Sun WY, Anthonymuthu TS, Tyurina YY, Kapralov O, Javadov S, He RR, Watkins SC, Winograd N, Kagan VE, Bayır H. (2021) Direct Mapping of Phospholipid Ferroptotic Death Signals in Cells and Tissues by Gas Cluster Ion Beam Secondary Ion Mass Spectrometry (GCIB-SIMS). Angew Chem Int Ed Engl. 2021 Mar 8. doi: 10.1002/anie.202102001. PMID: 33684237
- Kuznetsov AV, Javadov S, Margreiter R, Grimm M, Hagenbuchner J, Ausserlechner MJ. (2021) Structural and functional remodeling of mitochondria as an adaptive response to energy deprivation. Biochim Biophys Acta Bioenerg 1862: 148393. PMID: 33549532
- Javadov S, Jang S, Chapa-Dubocq XR, Khuchua Z, Camara AKS. (2021) Mitochondrial respiratory supercomplexes in mammalian cells: structural versus functional role. J Mol Med (Berl), 99:57-73. PMID: 33201259
- Rodríguez-Graciani KM, Chapa-Dubocq XR, MacMillan-Crow LA, Javadov S. (2020) Association between L-OPA1 cleavage and cardiac dysfunction during ischemia-reperfusion injury in rats. Cell Physiol Biochem 54:1101-1114. PMID: 33119220
- Javadov S, Kozlov AV, Camara AKS. (2020) Mitochondria in health and diseases. Cells, 9: 1177. PMID: 32397376
- Chapa-Dubocq X, Rodriguez-Graciani KM, Guzmán-Hernández R, Jang S, Brookes PS, Javadov S. (2020) Cardiac function is not susceptible to moderate disassembly of mitochondrial respiratory supercomplexes. J. Mol. Sci. 21:1555. PMID: 32106430
- Kuznetsov AV, Javadov S, Grimm M, Margreiter R, Ausserlechner MJ, Hagenbuchner J (2020). Intracellular crosstalk between mitochondria and cytoskeleton in cardiac and skeletal muscles. Cells, 9: 222. PMID: 31963121
- Jang S, Javadov S. (2020) OPA1 regulates respiratory supercomplexes assembly: the role of mitochondrial swelling. 51:30-39. PMID: 31870826
- Makarov V, Khmelinskii I, Khuchua Z, Javadov S. (2020) In silico simulation of reversible and irreversible swelling of mitochondria: the role of membrane rigidity. 50:71-81. PMID: 31669621
- Parodi-Rullán RM, Chapa-Dubocq X, Guzmán-Hernández R, Jang S, Torres-Ramos CA, Ayala-Peña S, Javadov S. (2019) The role of adenine nucleotide translocase in the assembly of respiratory supercomplexes in cardiac cells. 8: 1247. PMID: 31614941.
- Kuznetsov AV, Javadov S, Margreiter R, Grimm M, Hagenbuchner J, Ausserlechner MJ. (2019). The Role of mitochondria in the mechanisms of cardiac ischemia-reperfusion injury. Antioxidants (Basel). 8: pii: E454. PMID: 31590423
- Escobales E, Nuñez RE, Javadov S. (2019) The intracellular renin-angiotensin system and mitochondria: implications in cardioprotective pathways. Am J Physiol Heart Circ Physiol, 316: H1426-H1438. PMID: 30978131.
- Jang S, Javadov S. (2018) Elucidating the contribution of ETC complexes I and II to the respirasome formation in cardiac mitochondria. Sci Rep, 8: 17732. PMID: 30531981.
- Khuchua Z, Glukhov AI, Strauss AW, Javadov S. (2018) Elucidating the beneficial role of PPAR agonists in cardiac diseases. Int J Mol Sci, 19: E3464. PMID: 30400386
- Parodi-Rullán RM, Soto-Prado J, Vega-Lugo J, Chapa-Dubocq X, Díaz-Cordero SI, Javadov S. (2018) Elucidating the cardioprotective effects of cyclophilin-D inhibition in female rats: acute vs. chronic post-myocardial infarction. Cell Physiol Biochem, 50:288-303. PMID: 30282073
- Parodi-Rullán RM, Chapa-Dubocq X, Javadov S. (2018) Acetylation of mitochondrial proteins in the heart: the role of SIRT3. Front Physiol. 9:1094. PMID: 30131726
- Nuñez RE, Javadov S, Escobales E. (2018) Critical role of angiotensin II type 2 receptors in the control of mitochondrial and cardiac function in angiotensin II-preconditioned rat hearts. Pflugers Arch. 470:1391-1403 PMID: 29748710
- Schafer C, Moore V, Dasgupta N, Javadov S, James JF, Glukhov AI, Strauss AW, Khuchua Z. (2018) The effects of PPAR stimulation on cardiac metabolic pathways in Barth syndrome mice. Front Pharmacol. 9: 318. PMID: 29695963
- Jang S, Javadov S. (2018) Current challenges in elucidating respiratory supercomplexes in mitochondria: methodological obstacles. Front Physiol. 9: 238. PMID: 29615931
- Makarov V, Khmelinskii I, Javadov S. (2018) Computational modeling of in vitro swelling of mitochondria: a biophysical approach. Molecules, 23: 783. PMID: 29597314
- Chapa-Dubocq X, Makarov V, Javadov S. (2018) Simple kinetic model of mitochondrial swelling in cardiac cells. J Cell Physiol. 233:5310-5321. PMID: 29215716
- Javadov S, Chapa-Dubocq X, Makarov V (2018) Different approaches to modeling analysis of mitochondrial swelling. 38: 58-70. PMID: 28802667
- Jang S, Javadov S. (2017) Association between ROS production, swelling and the respirasome integrity in cardiac mitochondria. Arch Biochem Biophys. 630: 1-8. PMID: 28736227
- Nuñez RE, Javadov S, Escobales E. (2017) Angiotensin II-preconditioning is associated with increased PKCε/PKCδ ratio and prosurvival kinases in mitochondria. Clin Exp Pharmacol Physiol, 44:1201-1212. PMID: 28707739
- Parodi-Rullán RM, Chapa-Dubocq X, Rullán PJ, Jang S, Javadov S. (2017) High sensitivity of SIRT3 deficient hearts to ischemia-reperfusion is associated with mitochondrial abnormalities. Front Pharmacol. 8:275. PMID: 28559847
- Javadov S, Jang S, Parodi-Rullan R, Khuchua Z, Kuznetsov AV. (2017) Mitochondrial permeability transition in cardiac ischemia-reperfusion: whether cyclophilin D is a viable target for cardioprotection? Cell Mol Life Sci. 74:2795-2813. PMID: 28378042
- Kuznetsov AV, Javadov S, Saks V, Margreiter R, Grimm M. (2017) Synchronism in mitochondrial ROS flashes, membrane depolarization and calcium sparks in human carcinoma cells. Biochim Biophys Acta Bioenerg 1858:418-431. PMID: 28279675
- Huang Y, Powers C, Moore V, Schafer C, Ren M, Phoon CK, James JF, Glukhov AV, Javadov S, Vaz FM, Jefferies JL, Strauss AW, Khuchua Z. (2017) The PPAR pan-agonist bezafibrate ameliorates cardiomyopathy in a mouse model of Barth syndrome. Orphanet J Rare Dis. 12:49. PMID: 28279226
- Jang S, Lewis TS, Powers C, Khuchua Z, Baines CP, Wipf P, Javadov S. (2017) Elucidating mitochondrial ETC supercomplexes in the heart during ischemia-reperfusion. Antioxid Redox Signal. 27: 57-69. PMID: 27604998
- Rodríguez-Reyes N, Rodríguez-Zayas AE, Javadov S, Frontera WR. (2016) Single muscle fiber contractile properties in diabetic rat muscle. Muscle Nerve. 53:958-64. PMID: 26598963
- Javadov S, Jang S, Rodriguez-Reyes N, Rodriguez-Zayas AE, Soto Hernandez J, Krainz T, Wipf P, Frontera W. (2015) Mitochondria-targeted antioxidant preserves contractile properties and mitochondrial function of skeletal muscle in aged rats. 6:39469-81. PMID: 26415224
- Javadov S, Escobales E. (2015) The role of SIRT3 in mediating cardioprotective effects of RAS inhibition on cardiac ischemia-reperfusion. J Pharm Pharm Sci. 18: 547-50. PMID: 26517140
- Huang Y, Powers C, Madala SK, Greis KD, Haffey WD, Towbin JA, Purevjav E, Javadov S, Strauss AW, Khuchua Z. (2015) Cardiac metabolic pathways affected in the mouse model of Barth syndrome. PLoS One, 10: e0128561; PMID: 26030409
- Zhdanov DD, Fahmi T, Wang X, Apostolov EO, Sokolov NN, Javadov S, Basnakian AG. (2015) Regulation of apoptotic endonucleases by mitochondrial EndoG. DNA Cell Biol. 34:316-26; PMID: 25849439
- Javadov S (2015). The calcium-ROS-pH triangle and mitochondrial permeability transition: challenges to mimic cardiac ischemia-reperfusion. Front Physiol. 6:83; PMID: 25852570
- Barreto-Torres G, Soto Hernández J, Jang S, Rodríguez-Muñoz AR, Torres-Ramos CA, Basnakian AG, Javadov S. (2015) The beneficial effects of AMP-kinase activation against oxidative stress are associated with prevention of PPARα/cyclophilin D interaction in cardiomyocytes. Am J Physiol Heart Circ Physiol. 308:H749-H758; PMID: 25617357
- Kuznetsov AV, Javadov S, Sickinger S, Frotschnig S, Grimm M. (2015). H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim Biophys Acta. 1853: 276–284; PMID: 25450968
Complete List of Published Work:


